Work
in my laboratory is supported by NIH grants from the National Institute
on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on
Drug Abuse (NIDA). A brief summary of some of the projects supported by
each grant are discussed below. Feel free to contact me for additional information.
1.NIAAA grant R37 AA009986. This grant is
focused on understanding the interactions of ethanol with NMDA receptors
 NMDA
receptors are ion channels activated by glutamate and are composed of
multiple subunits
(GluN1, GluN2 and GluN3) that possess extracellular, transmembrane, and
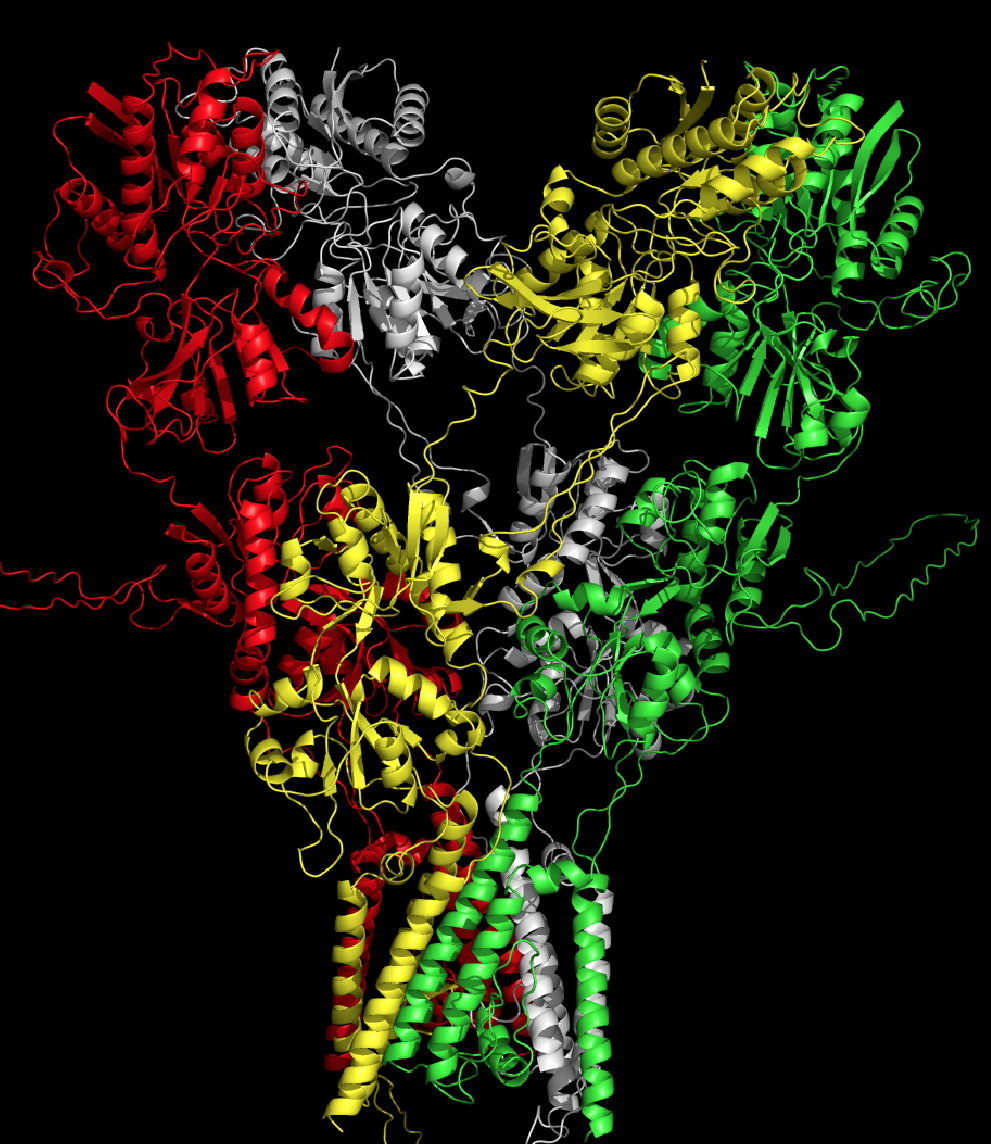
intracellular domains. The figure to the left shows the GluN1/GluN2A
receptor. This structure was modeled after the GluA2 crystal structure
of Sobolevsky et al. (2009) and is from our recent study by Xu et al.
(2012). Our previous work showed that alcohol
inhibits the function of NMDA receptors. The major question is
how and where?
NMDA
receptors are ion channels activated by glutamate and are composed of
multiple subunits
(GluN1, GluN2 and GluN3) that possess extracellular, transmembrane, and
intracellular domains. The figure to the left shows the GluN1/GluN2A
receptor. This structure was modeled after the GluA2 crystal structure
of Sobolevsky et al. (2009) and is from our recent study by Xu et al.
(2012). Our previous work showed that alcohol
inhibits the function of NMDA receptors. The major question is
how and where?
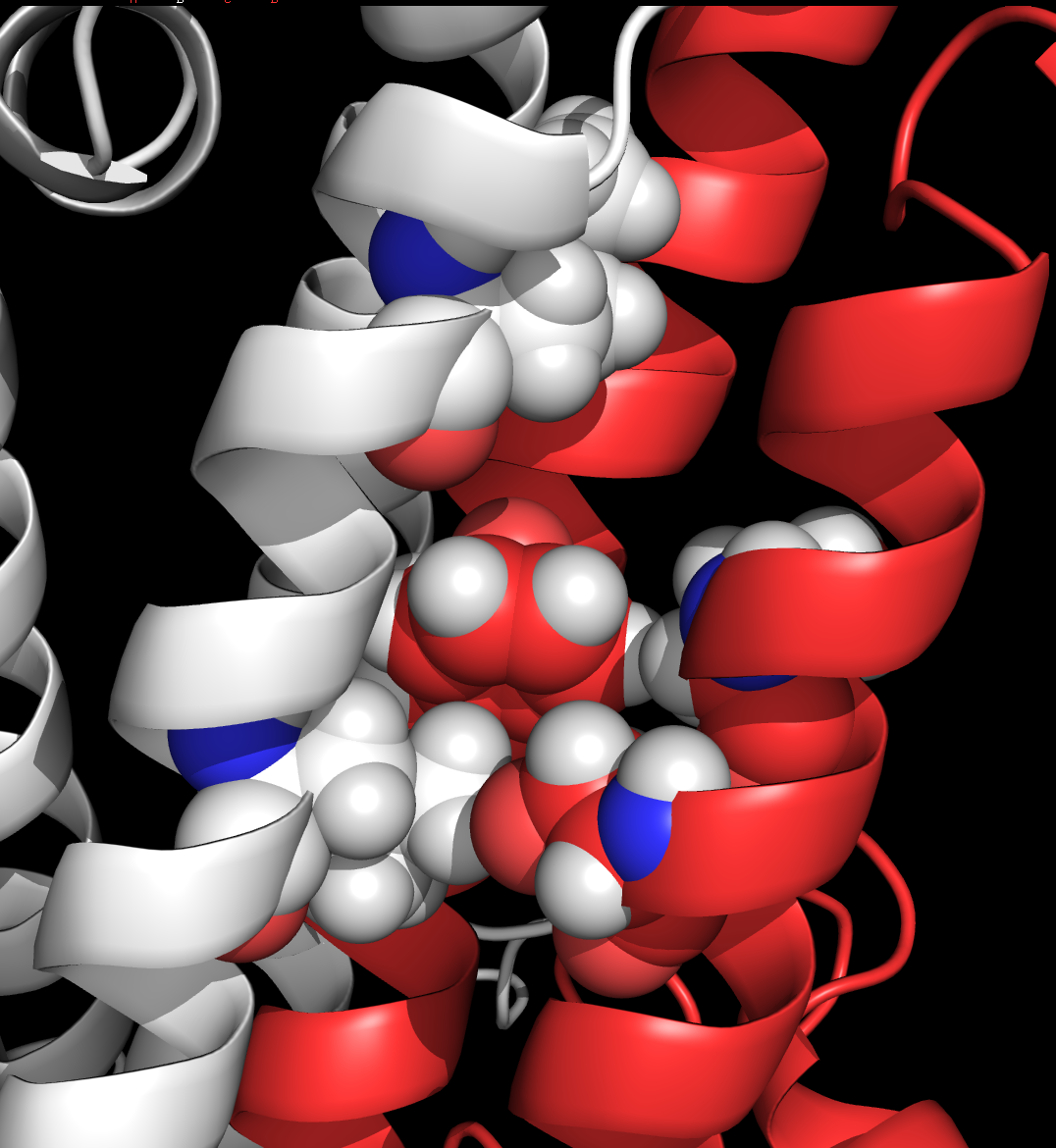
We began by
identifying
candidate residues in the transmembrane domains of the NR1 subunit that
could define an alcohol site of action. We substituted the small
alanine residue at selected locations and tested these mutant receptors
for function and alcohol sensitivity. As
described
in Ronald
et al., 2001 and Smothers and Woodward, 2006, subsituting alanine for
phenylalanine at position 639 in the third transmembrane domain of the
NR1 subunit reduces ethanol inhibition of the receptor.
Although expressing
mutant NMDA subunits in neurons is a useful approach, these subunits
must compete with wild-type subunits in order to produce an effect.
To eliminate this concern, we have
collaborated with Dr. Gregg Homanics at the University of Pittsburgh
to produce mice in which the genes that code for wild-type NMDA
receptor subunits are replaced with
those containing alcohol/anesthetic insensitive sites. In the
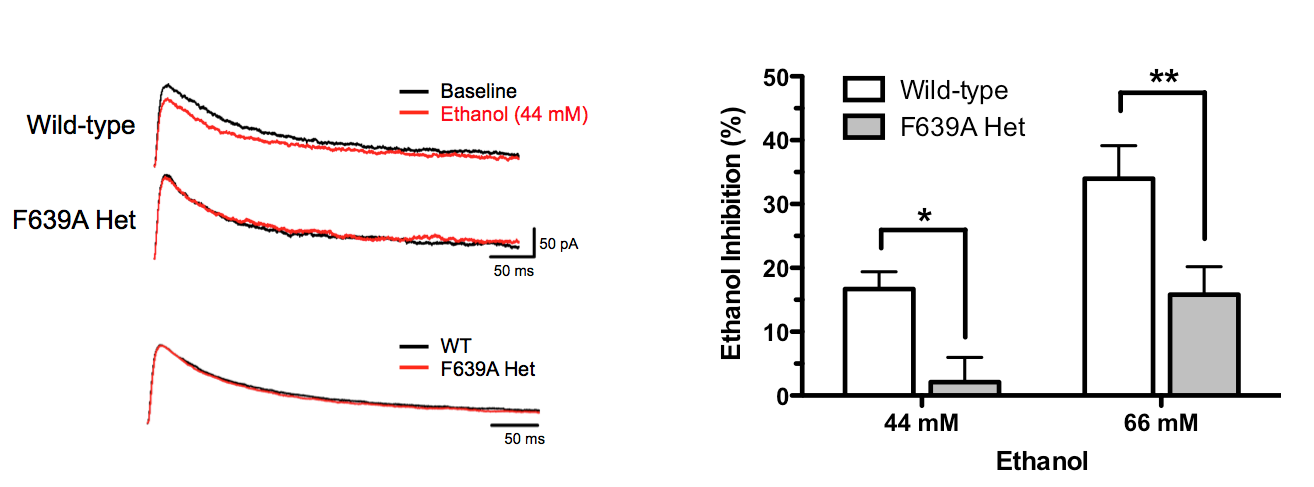
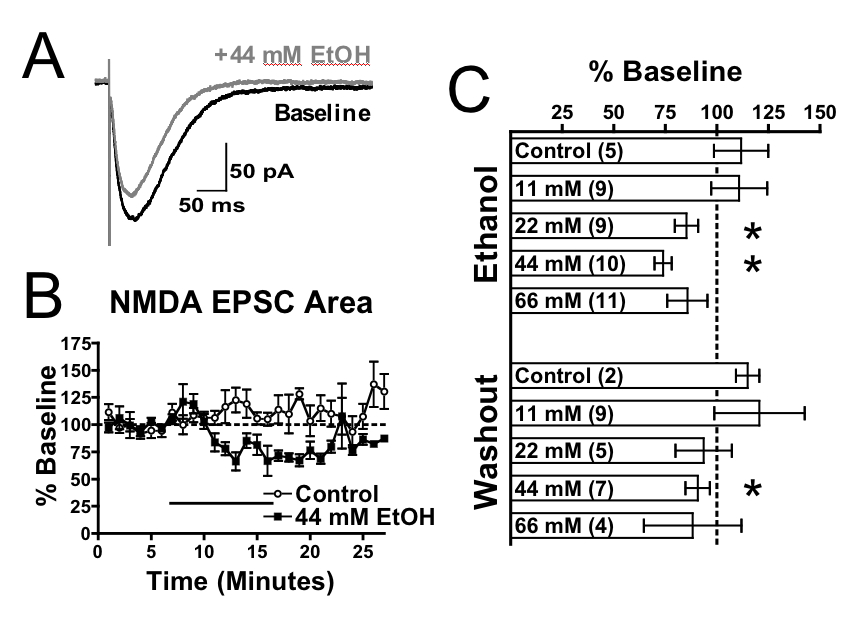
first report of these animals (den Hartog et al., PlosOne 2013), mice
carrying the GluN1 (F639A) mutation showed reduced ethanol inhibition
of NMDA EPSCs in mPFC neurons (left figure) and displayed alterations
in selected behaviors.
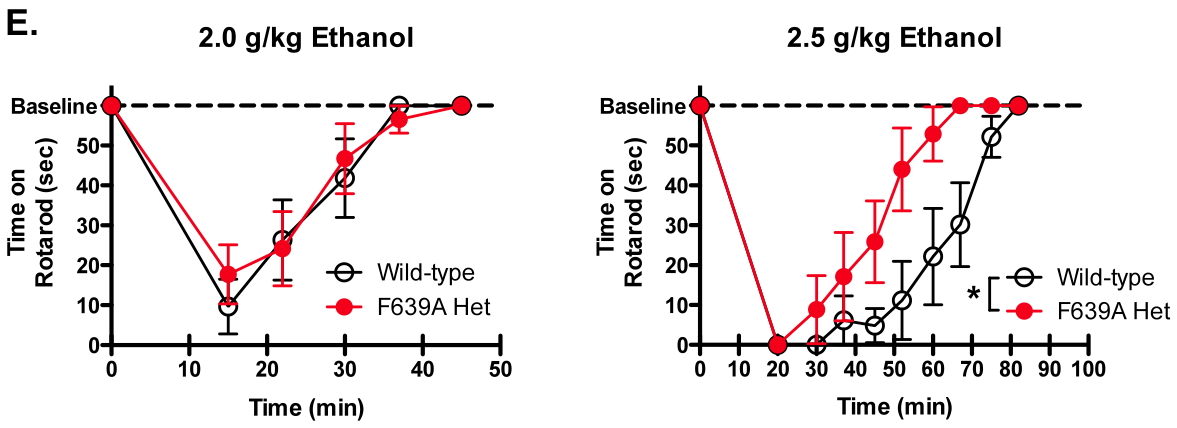
These included loss
of locomotor activation at low ethanol doses, faster recovery from ethanol-induced impairment of motor function on
the rotarod (left figure), reduced anxiolytic effects of acute ethanol
on the elevated zero-maze, and enhanced
drinking in the intermittentaccess model. No changes were observed in
ethanol-induced loss of righting reflex or sleep time, hypothermia or ethanol metabolism.
2.
NIDA Grant R01DA13951. This grant supports research into
the neural actions of toluene; a prototypic member of the class of
abused inhalants. Inhalant abuse is prevalent among children and
adolescents and is an under-studied area of addiction neuroscience.
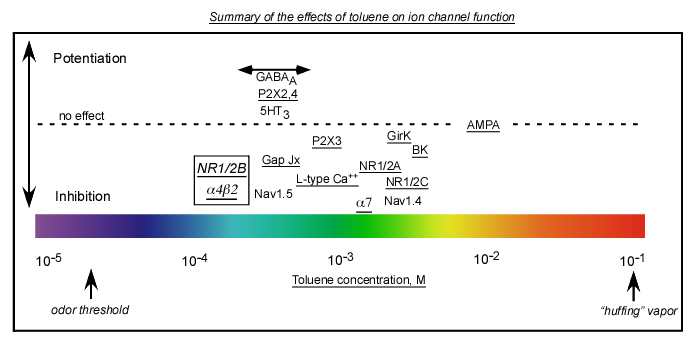
A major project in the lab
is to determine which brain ion channels
are sensitive to solvents such as toluene and how this alters the
function of neurons in key brain regions involved in addiction. The
cartoon above summarizes our current understanding of the effects of
toluene on the major classes of ion channels (underlined channels
indicates work done in our lab). Channels are positioned from left
(more sensitive) to right (less sensitive) and above (potentiated) or
below (inhibited) the dashed line to indicate their relative
sensitivity to toluene.
A) Voltage-gated ion channels-Calcium channels (L and N type), Sodium channels (work by Cruz), Potassium channels (BK, GirK)
B) Glutamate ion channels-NMDA (2B most sensitivr, 2C least), AMPA (no effect except at very high concentrations)
C) GABA/Glycine ion channels-Most potentiated (Work by Mihic and colleagues)
D) ATP ion channels-P2X2,4 subtypes potentiated; P2X3 inhibited
E) Acetylcholine ion channels-Alpha4/Beta2 most sensitive, Alpha7 least
 We
have also examined the effects of toluene on neurons
within the addiction neurocircuitry of the brain. These studies
focus on neurons within the prefrontal cortex, ventral tegmental area
(VTA) and nucleus accumbens and use whole-cell patch clamp
electrophysiology to quantitate solvent effects on glutamatergic and
GABAergic synaptic transmission.
We
have also examined the effects of toluene on neurons
within the addiction neurocircuitry of the brain. These studies
focus on neurons within the prefrontal cortex, ventral tegmental area
(VTA) and nucleus accumbens and use whole-cell patch clamp
electrophysiology to quantitate solvent effects on glutamatergic and
GABAergic synaptic transmission.
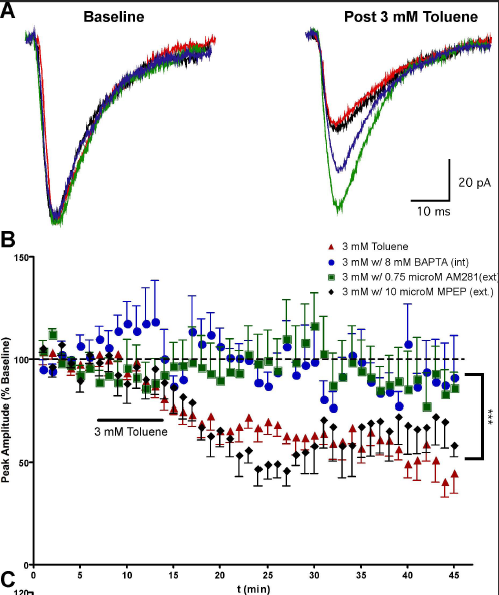
The results
show that toluene induces
a long-lasting depression of AMPA EPSCs in PFC pyramidal neurons (left; Beckley and Woodward, 2011) and NAc medium spiny neurons (right, Beckley et al., 2015)
and that this effect is mediated via the endocannabinoid system.
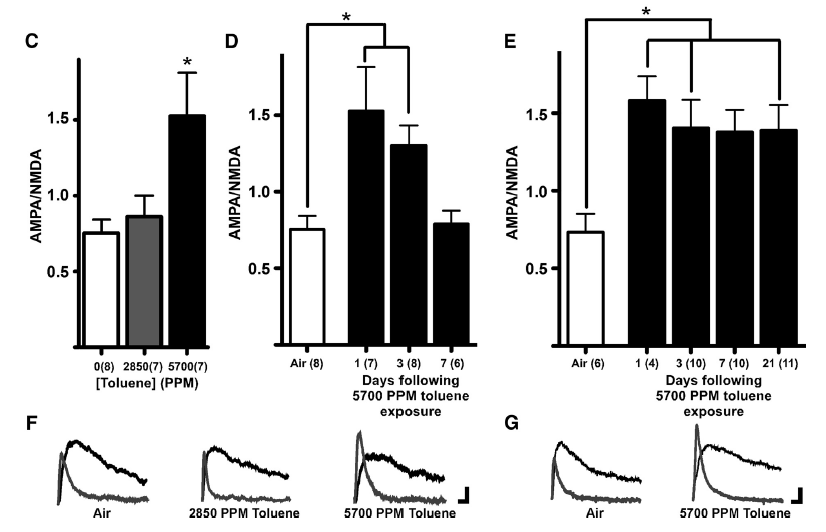 Using a vapor model of inhalation, we have also
shown that one exposure to toluene vapor induces a long-lasting
increase in the AMPA/NMDA ratio of VTA DA neurons (Beckley et al., 2013). This is restricted to
DA neurons that project to the nucleus accumbens as no change was observed in VTA DA neurons
that project to the mPFC. In addition, the toluene-induced change in VTA DA neuron AMPA/NMDA ratio
could be reversibly controlled by regulating the output of the mPFC during toluene exposure.
Using a vapor model of inhalation, we have also
shown that one exposure to toluene vapor induces a long-lasting
increase in the AMPA/NMDA ratio of VTA DA neurons (Beckley et al., 2013). This is restricted to
DA neurons that project to the nucleus accumbens as no change was observed in VTA DA neurons
that project to the mPFC. In addition, the toluene-induced change in VTA DA neuron AMPA/NMDA ratio
could be reversibly controlled by regulating the output of the mPFC during toluene exposure.
3. NIAAA funded Charleston Alcohol Research Center (ARC; P50AA10761).
Effects of ethanol on network activity in the
prefrontal cortex
While
neurons in brain slices acutely isolated from experimental animals
retain the anatomical connections that exist in the intact brain,
activity in these slices is often lost due to disruption of normal
inputs. To better simulate the activity patters of the intact
PFC, we have adapted a novel slice culture preparation that maintains
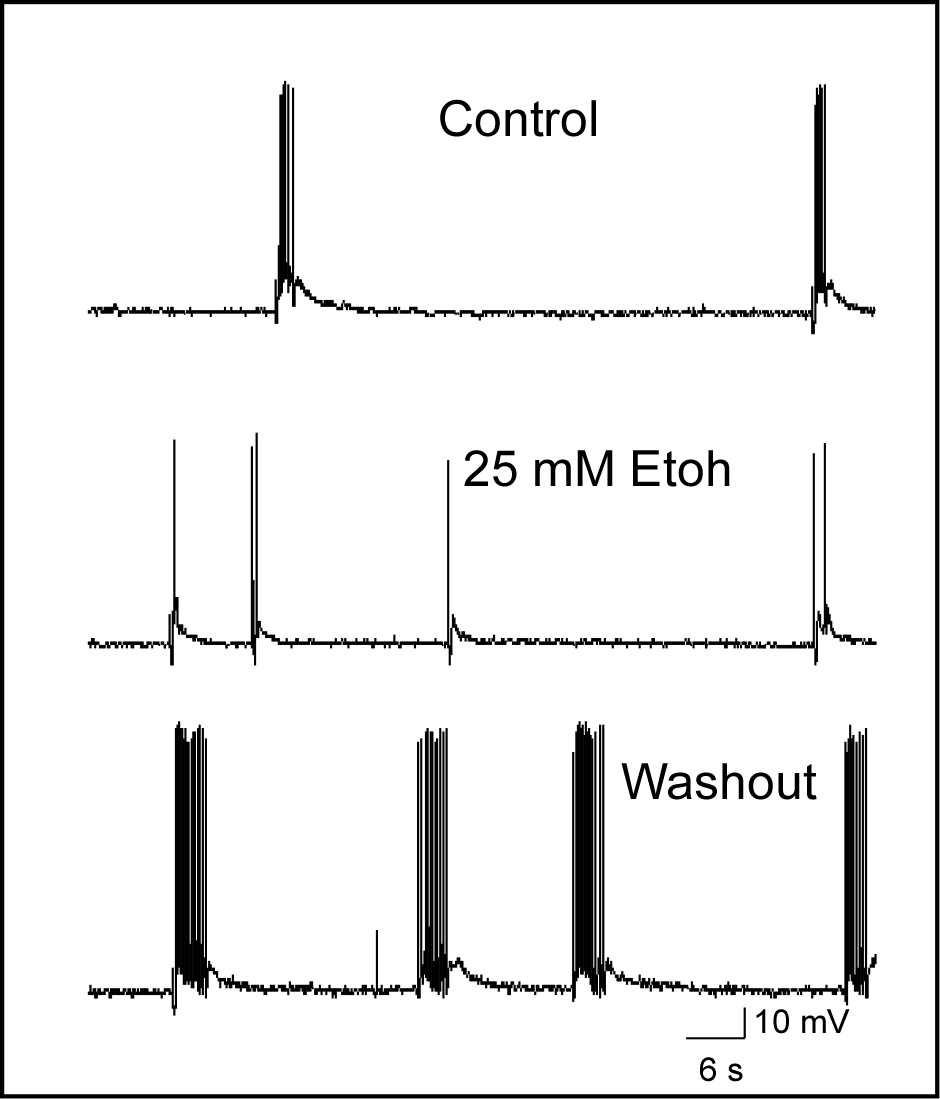
normal patterns of activity. For example, shown in the figures
below are traces
from deep-layer pyramidal neurons from the prefrontal cortex
that are maintained in a novel triple slice co-culture containing
slices of
cortex, hippocampus and midbrain (left panel). These neurons
display
spontaneous and evoked periods of persistent activity characterized by
sudden plateau depolarizations (up-states) accompanied by spike firing.
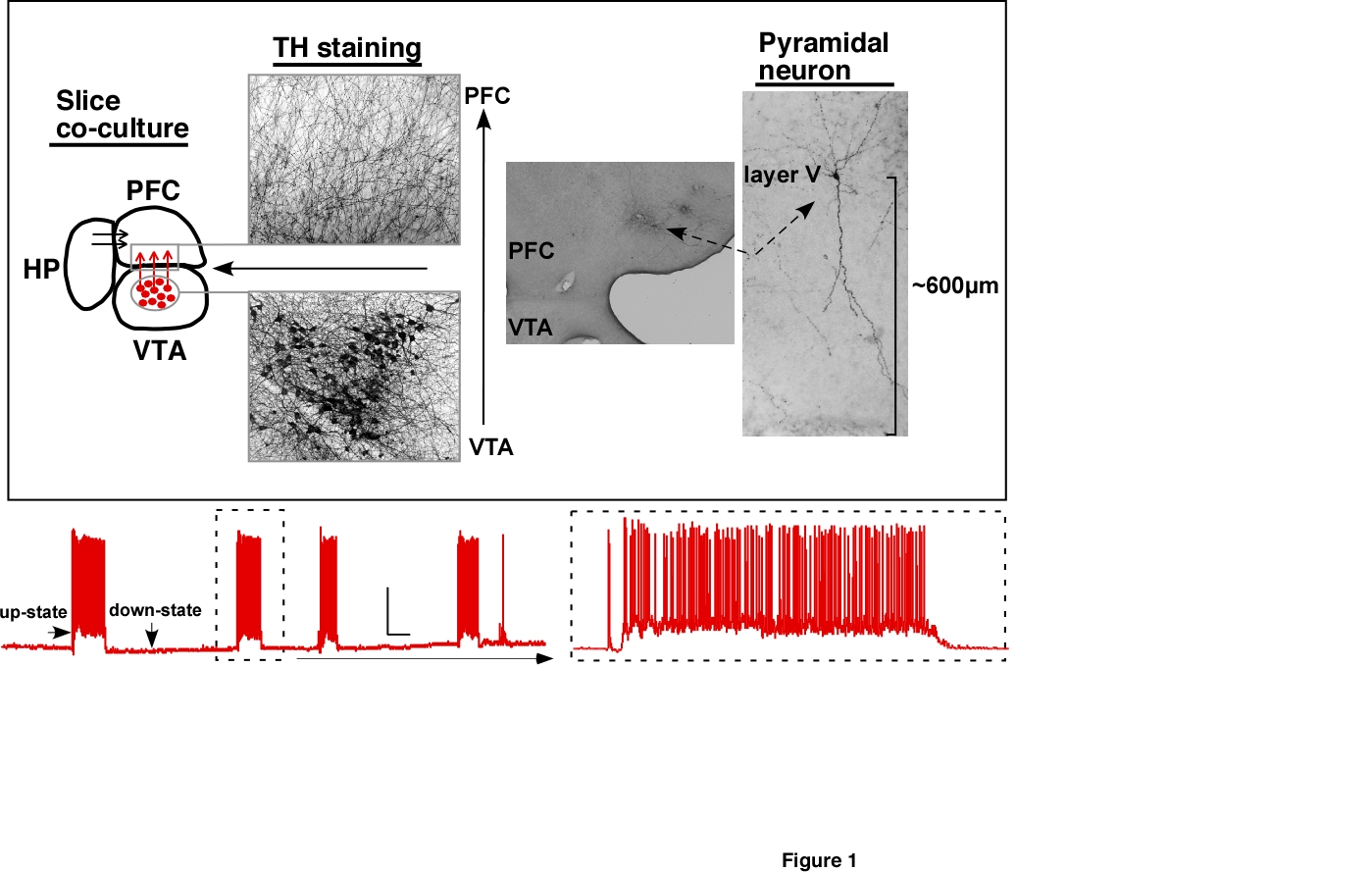
As reported in previously published papers (Tu
et al., 2007; Weitlauf and
Woodward, 2008; Woodward and Pava, 2009), ethanol inhibits these
up-states and following
washout,
neurons often display an enhanced period of persistent activity. The
inhibition of persistent activity appears to result from block of
synaptic NMDA receptors as ethanol had no effects on AMPA or
GABA-mediated currents in mPFC pyramidal neurons (Weitlauf et al.,
2008).
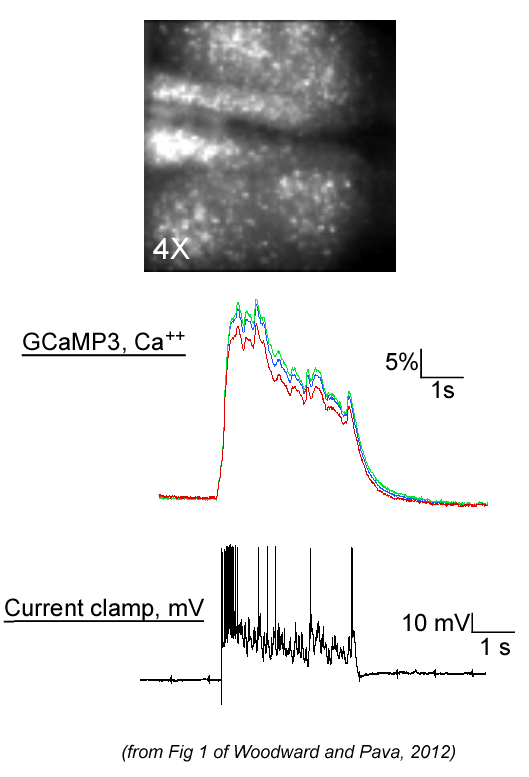
In addition to electrophysiological
analysis of persistent activity, we published a study
(Woodward and Pava, 2011; ACER) that used a fast image acquisition
system (
RedShirt NeuroCCD)
to monitor up-states in slice cultures that express a calcium reporter
protein.
The Quicktime movies listed show examples of multi-neuron
activity during stimulus-evoked up-states in our prefrontal cortical slice cultures.
Full-field images were acquired at frame rates of 40-125 Hz and were collected at either
40X or 20X
magnification. Movies run at 50 frames/sec. We are
currently using this system to examine the effects of ethanol on
network patterns of persistent activity and up-states.
(Tiffstack340X.mov) (Tiffstack20X.mov).
We have also examined the role of endocannabinoids
in regulating mPFC persistent activity and linked these actions to the role
of these modulators in sleep (Pava et al., 2014). We also examined how
chronic exposure to ethanol alters mPFC persistent activity and showed that
up-state duration but not amplitude are enhanced following 10 days exposure to
44 mM ethanol (Pava and Woodward, 2014). In addition, while CB1R agonists increased the amplitude of
up-states under control conditions, these agents had no effect on up-states in ethanol-treated
neurons even in the absence of a change in CB1 receptor expression.
Effects of ethanol on OFC neurons and behavior
In a related and ongoing study supported by the ARC, we are determining the effects of
acute and
chronic ethanol on the function of neurons in the orbitofrontal cortex.
The OFC receives inputs from all major sensory systems and is
critically involved in assigning value to both food and actions.
Damage to the OFC is associated with deficits in the ability to
determine reward value and patients with such damage often show risky
behavior and problems learning new rules that normally allow one to
adapt to new situations.
In these studies, whole-cell
patch-clamp
electrophysiology and behavioral assessments are combined with a vapor
inhalation model that produces ethanol dependence in mice.
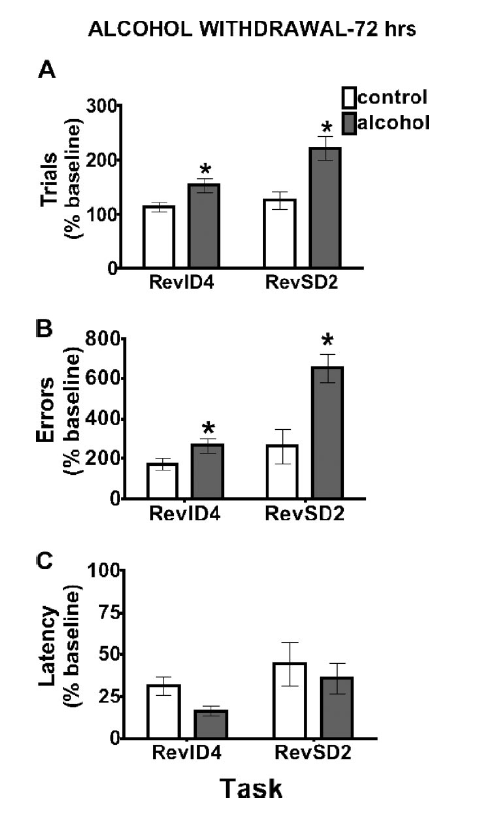
The figure to the left is from Badanich et al., 2011 and
shows that mice that had undergone multiple cycles of ethanol
intoxication and withdrawal showed deficits (increased trials to
criterion, increased errors) in a task that measures reversal
learning. The figure on the right is from Badanich et al. (2013)
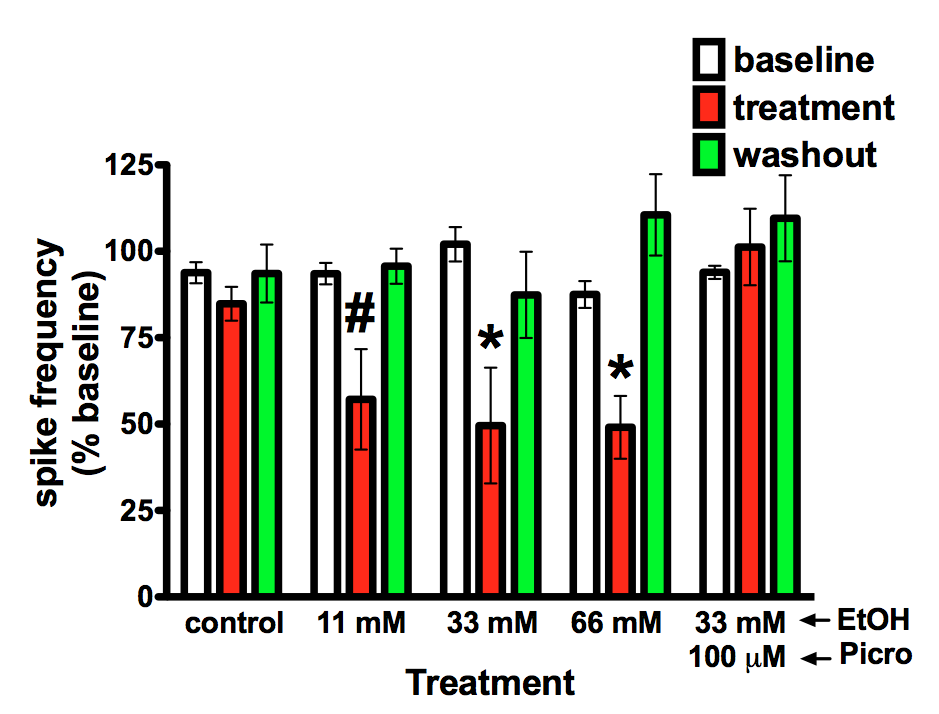
and shows that ethanol reduces current evoked spiking at relatively low
concentrations. Follow-up studies presented in that paper showed that
this effect was mediated by a novel strychnine sensitive process thus
implicating glycine receptors in this action. Our current work is
investigating how chronic exposure to ethanol alters OFC neuron
excitability and affects ethanol's ability to reduce firing.
back to homepage

 NMDA
receptors are ion channels activated by glutamate and are composed of
multiple subunits
(GluN1, GluN2 and GluN3) that possess extracellular, transmembrane, and
intracellular domains. The figure to the left shows the GluN1/GluN2A
receptor. This structure was modeled after the GluA2 crystal structure
of Sobolevsky et al. (2009) and is from our recent study by Xu et al.
(2012). Our previous work showed that alcohol
inhibits the function of NMDA receptors. The major question is
how and where?
NMDA
receptors are ion channels activated by glutamate and are composed of
multiple subunits
(GluN1, GluN2 and GluN3) that possess extracellular, transmembrane, and
intracellular domains. The figure to the left shows the GluN1/GluN2A
receptor. This structure was modeled after the GluA2 crystal structure
of Sobolevsky et al. (2009) and is from our recent study by Xu et al.
(2012). Our previous work showed that alcohol
inhibits the function of NMDA receptors. The major question is
how and where? 









